Purpose: Dual expression of MYC and BCL2 proteins (Double Expressor Lymphoma-[DEL]) is an important prognostic factor in patients (pts) with diffuse large B-cell lymphoma (DLBCL) who are treated with standard chemo-immunotherapy. Studies have suggested that pts with these biomarkers have inferior survival compared to pts who were non-DEL after autologous stem cell transplantation (autoSCT). For this reason, allogeneic stem cell transplantation (alloSCT) has been advocated for its potential graft-versus-lymphoma effect. Data are limited however regarding the outcomes in pts with relapsed DEL who were treated with autoSCT vs. alloSCT.
Methods: Data from pts with relapsed DEL/DLBCL who underwent auto- and alloSCT as their first transplant at our center and in whom archived tumor material was available were analyzed. Cutoff values of 40% for MYC and 70% for BCL2 were established by immunohistochemistry (IHC). The majority of autoSCT pts (84%) underwent chemo-mobilization of stem cells with rituximab (R) for in-vivo purging; rituximab was also given on days+1 and +8 with BEAM conditioning (J Clin Oncol 2005; 23:2240-7; Clin Cancer Res 2018; 24:2304-11). Conditioning for the alloSCT was myeloablative (n=16, 50%), reduced-intensity (n=6, 19%), and nonmyelablative (n=10,31%). The study was IRB-approved at our center. Kaplan-Meier analysis and corresponding log-rank/Gray's tests were used to estimate and compare overall survival (OS), progression-free survival (PFS), and cumulative incidence (CI) of non-relapse mortality and relapse by SCT group. Standard multivariable Cox regression was used to evaluate associations between SCT type and OS/PFS after controlling for differences in baseline characteristics and cause-specific multivariable Cox regression was used for competing-risk outcomes [non-relapse mortality (NRM) and relapse].
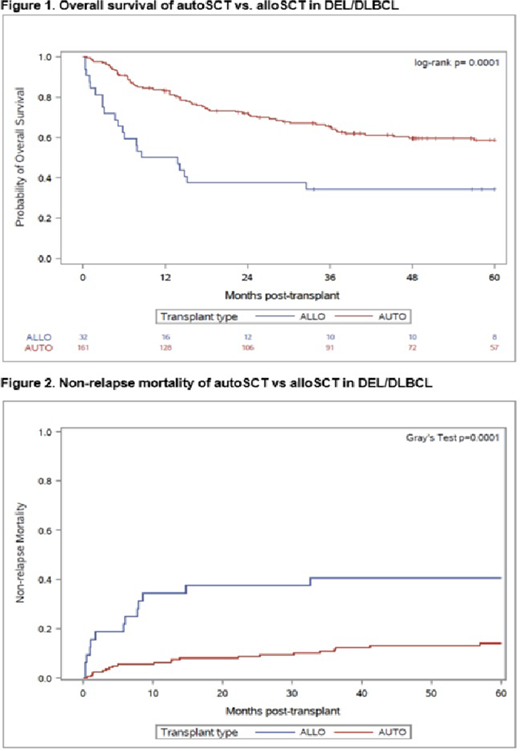
Results: The study included 161 autoSCT and 32 alloSCT pts treated between 2000-2018. Median age was 59 (range,18-80) and 55 years (range, 21-66), respectively (P=0.009). Male gender was 62.7 vs 50.0%, respectively (P= 0.234). Fifty-one (32.5%) autoSCT pts and 9 (28.1%) alloSCT pts had an elevated serum LDH (P= 0.683) and 41/148 (27.7%) autoSCT and 11/25 (44.0%) alloSCT pts were PET-positive at study entry (P=0.106). The time from diagnosis to transplant was 20.0 and 25.2 months, respectively, (P=0.068), and the distribution of the years of transplant was similar between both groups (P=0.317). Significant differences between treatment groups were observed for prior number of chemotherapies, disease status, stage, and HCT-CI score at transplant. Pts. receiving alloSCT were more heavily pretreated [median 3 prior therapies, (range 1-8) vs. a median of 2, (range 1-6); P=0.0001], had more advanced stage III-IV disease (P=0.035), and more refractory disease (P=0.010) at transplant. In addition, a higher percentage of alloSCT pts had an HCT-CI of >4 (28.1% vs. 10.2%, P=0.018) at study entry. Allogeneic transplant characteristics included matched siblings (n=18; 56.3%), matched unrelated (n=13, 40.6%) and haplo-identical (n=1, 3.1%) donors. With a median follow-up for autoSCT surviving pts of 65 months (range, 5-217 months) and 93 months (range, 34--124 months) for alloSCT pts, the OS at 5-years were 59% and 34%, respectively (P= 0.0001) (Figure1). The PFS rates at 5-years were 49% and 31%, respectively (P = 0.002). AutoSCT had a similar rate of relapse to alloSCT but a lower rate of NRM. The 5-year CI of relapse was 37% vs 28%, respectively (P = 0.611) and the 5-year NRM rates were 14% vs 41 % (P=0.0001), respectively (Figure 2). The associations between SCT group and outcomes persisted after adjusting for differences between groups (alloSCT vs. autoSCT HR= 3.46/p=0.0004 for OS; HR=2.67/p=0.005 for PFS; HR=4.74/p=0.009 for NRM; HR=2.22/p=0.080 for relapse). The 100-day CI of grade II-IV and III-IV acute GVHD in the alloSCT group was 37.5 and 9.4%, respectively. The 1-year CI of chronic GVHD was 25.0%.
Conclusions: This study is the first to show that autoSCT confers a superior survival in pts with relapsed DEL/DLBCL compared to alloSCT. Our conclusions are supported by long-term follow-up. The use of novel nonmyeloablative regimens in a larger number of pts receiving an alloSCT and ongoing studies at our center with targeted investigational agents after autoSCT may improve results.
Khouri:Pfizer: Research Funding; Bristol Myers Squibb: Research Funding. Iyer:CRISPR: Research Funding; Spectrum: Research Funding; Daiichi Sankyo: Consultancy; Trillium: Research Funding; Curio Biosciences: Honoraria; Target Oncology: Honoraria; Afffimed: Research Funding; Merck: Research Funding; Legend Biotech: Consultancy; Rhizen: Research Funding; Seattle Genetics, Inc.: Research Funding. Popat:Bayer: Research Funding; Novartis: Research Funding. Qazilbash:Angiocrine: Research Funding; Amgen: Research Funding; Bioclinica: Consultancy; Janssen: Research Funding; Bioline: Research Funding. Champlin:DKMS America: Membership on an entity's Board of Directors or advisory committees; Actinium: Consultancy; Johnson and Johnson: Consultancy; Omeros: Consultancy; Cytonus: Consultancy; Takeda: Patents & Royalties; Genzyme: Speakers Bureau.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal